Application process to run a study
The application process to apply to conduct a surveillance study through the BPSU is outlined below in this section. There are two parts to the application process, and this will be thoroughly reviewed by the Scientific Committee.
There is a two-stage peer review application procedure to successfully pass through in order to be able to begin conducting surveillance through the BPSU. Phase 1 (P1) is an outline application to establish if the study meets the BPSU criteria. Applications should be submitted on the Phase 1 application form which can be found as a downloadable document at the bottom of this page. If the Phase 1 application is accepted by the Committee, feedback will be given and a more detailed phase two application will be requested.
A full outline of the application process is detailed below.
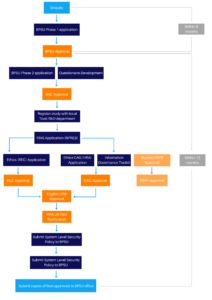
Phase 1 is the submission of an initial application to the BPSU Scientific Committee. If the Phase 1 application is accepted by the SC, a more detailed Phase 2 (P2) application will be invited.
Note: The Committee expects the submission of a Phase 2 application within six months of Phase 1 approval. If a phase 2 application is not forthcoming, the BPSU Office will contact the principal investigator to discuss the application.
If the Phase 1 application is approved by the committee, a more detailed Phase 2 application should be completed.
Alongside a completed Phase 2 application form, the Committee asks that you supply the following:
- Signed covering letter from the main contact/principal investigator for the study, which addresses any points raised by the SC in its phase one response letter
- Letters of support that you consider relevant for the SC to consider, for example award letters from funding bodies or letters confirming support by collaborating partners such as paediatric specialty groups
- Questionnaires, proformas and covering letters that will be used within the study – please provide a version number and date for each
- Data analysis plan, with a breakdown of how the questions are to be analysed and how they will address the objectives of the study – examples are available on request
- Public information materials, such as a leaflet
letters of support from supporting organisations, if appropriate.
If the Phase 2 application is approved, REC (Research Ethics Committee) and CAG-HRA (Confidentiality Advisory Group – Health Research Authority), PBPP (Public Benefit and Privacy Panel) approval should be obtained by the principal researcher and the IG (Information Governance) toolkit completed.
Note: Once a Phase 2 application has been accepted, the Committee expects the study to commence surveillance within 12 months of the Chair’s approval. If surveillance has not commenced within 12 months of the Chair’s approval, the BPSU Office will contact the principal investigator to discuss the application and the reasons for the delay. If a satisfactory response is not made, the BPSU reserves the right to revoke its phase two approval. The study team will be invited to ‘re-submit’ a phase 1 application.
Contributions
The current contributions for running a study through the BPSU is £20,000 for the first year (13 months) and £15,000 a year for subsequent years*.
Application submission dates
Phase one and two applications need to be submitted no later than three weeks before a BPSU Scientific Committee meeting. The dates of this year’s SC meetings are listed below:
| 29th January 2025 | 18th September 2025 |
| 20th March 2025 | 12th November 2025 |
| 23rd May 2025 |